The Yoshihara Laboratory focuses on the interdisciplinary fields of developmental biology, physiology, and genomics. The lab aims to uncover the transcriptional and epigenetic gene-regulatory networks underlying human islet organogenesis, tissue regeneration, cellular communication, and metabolic regulation, with the goal of identifying novel therapeutic candidates for diabetes. Using a multidisciplinary approach, the team investigates the molecular basis of organ development and metabolic regulation through advanced omics technologies, physiological models, and human organoids, while also advancing research in artificial intelligence (AI) and tissue engineering to enhance therapeutic development.Human pancreatic islet organogenesis
Human islet transplantation confers a significant improvement in glycemic control and prevents life-threatening severe hypoglycemia in type 1 diabetes (T1D) and late-stage of type 2 diabetic (T2D) patients. However, there are two limitations to this approach: 1) the chronic shortage of human islets; and 2) immunosuppression, which is associated with an increased risk of cancer and infections, is required to avoid rejection of transplanted islets. Together, these limitations create an unmet medical need. We investigate the mechanism underlying generation of immune evasive fully functional human pancreatic islets from human pluripotent stem cells (hPSCs). If we can create universally immune-tolerant, fully functional glucose-responsive human pancreatic islets from infinite cell resources, such as human pluripotent stem cells, this technology will benefit all who suffer from T1D and severe T2D, regardless of patient age, sex or genetic background. To the end, we study about human islet organogenesis by using in vitro organoids models created by hPSCs. During the development, lineage specification toward endocrine islet cell types (Insulin producing β cells, glucagon producing α cells, somatostatin producing δ cells etc) and maturation (Enhancing glucose sensitivity/mitochondrial metabolism) is required for functional islets. We study how cell fate determination and functionality is regulated by transcriptional factors and related environmental cues. In addition, we study the fundamental mechanism how functional β cells can be transformed immune evasive status through epigenetic memory system.
Key Technology; Human pluripotent stem cells, human islet-like organoids, Genome Engineering, Hormone Secretion Assay, bulk RNA-seq, scRNA-seq, ATAC-seq, Cut & Tag, Spatial Genes Expression, islet transplantation
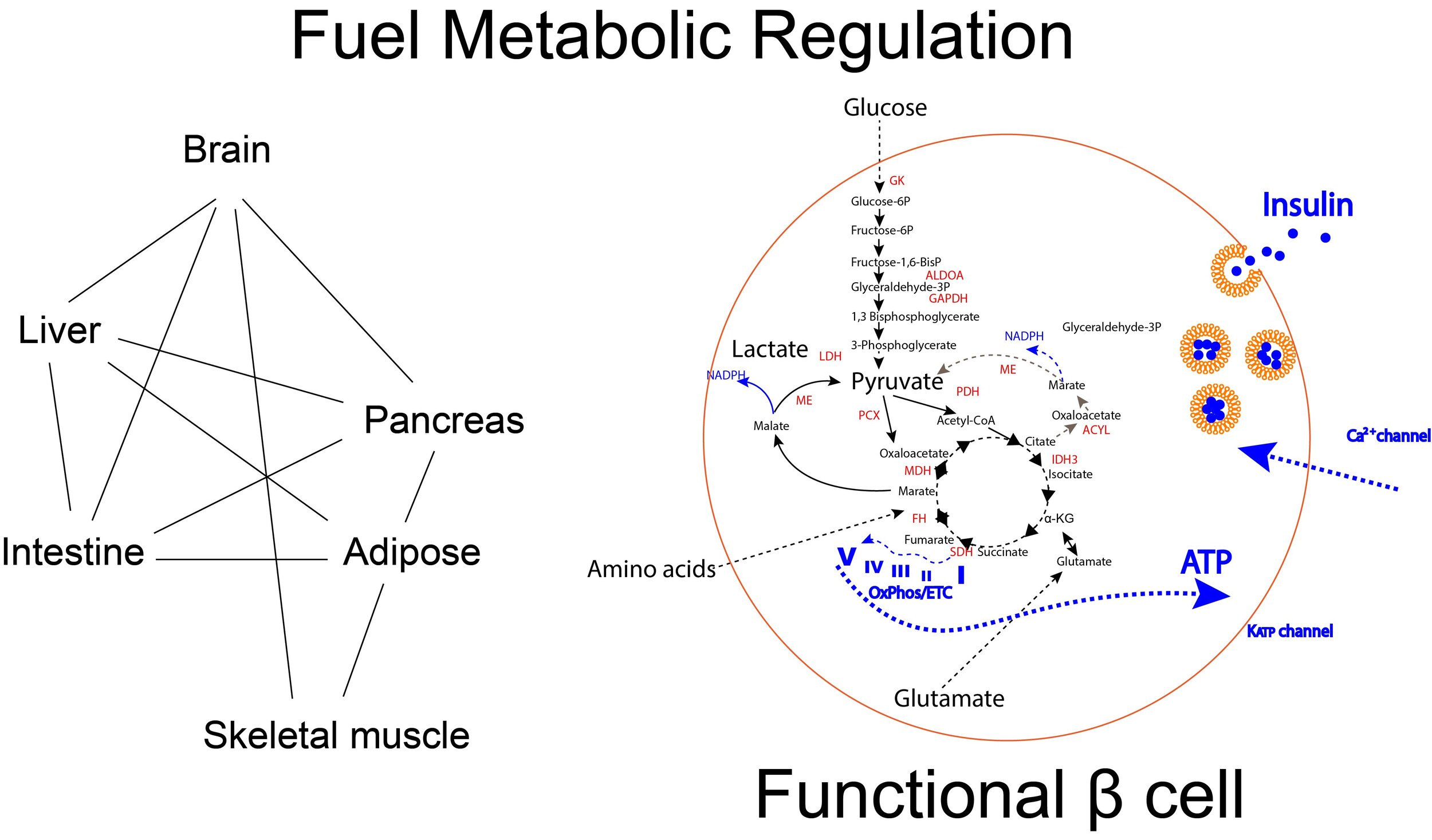
Whole body metabolic regulation, autoimmune reaction and pathogenesis of diabetes
Diabetes is a complex disease that affects more than 400 million people in the worldwide. In particular, type 1 diabetes (T1D) and late-stage of type 2 diabetes (T2D) is a significant burden that typically appears during adolescence and requires life-long administration of insulin and blood glucose monitoring. Currently treatment to ameliorate diabetes is available but still far-reaching for functional cure to relief patients from disease progression and daily treatment. Understanding of whole body metabolic regulation benefits to not only for therapeutics for diabetes and related complication but also understandings for nature of life. Whole body metabolic regulation is achieved by the collaboration of multiple organs and tissue resident cells through hormones, cytokines, proteins, circulated RNAs and metabolites or direct physical contact including nerves system. By using, genetic and disease animal models combining with multiomics analysis, we aim to elucidate the molecular mechanism of pathogenesis of T1D/T2D and related complication. High throughput drug screening technology will be used to identify the novel therapeutic candidates.
Key Technology; Islet Isolation and physiological assays, in vitro metabolic analysis, Immune profiling, Metabolomics, Protein-Protein interaction, Drug Screening
Bioengineering multi organ interaction
We generate many different type of human cell types/mini organs from pluripotent stem cells and adult stem cells. We are developing the tools to facilitate our understandings of cell-cell and organ-organ communication in human context. Multi discrepancy technology including, bioengineering, informatics and material science is used. We also look for the biofabrication, angiogenic-immune modulating materials which we can combine with stem-cell based cell therapy.
Key Technology; Organ-on-a-chip, microfluidics, biofabrication, co-culturing, robotics, informatics, nanoparticles